Metal-free regioselective tandem synthesis of diversely substituted benzimidazo-fused polyheterocycles in aqueous medium†
Abstract
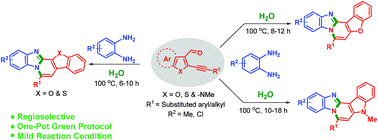
An operationally simple approach for the synthesis of diversely substituted benzimidazo-fused polyheterocycles under metal-free conditions in water has been described. The aqueous medium accelerates the formation of the desired product by avoiding side-products as well as harsh reaction conditions. The reaction proceeds through inter and intramolecular C–N bond formation in one-pot via 6-endo-dig cyclization. Deuterium labelling and X-ray crystallographic studies supported the proposed mechanistic pathway for the targeted cyclized product.

 Please wait while we load your content...
Please wait while we load your content...