Construction of the di(trimethylolpropane) cross linkage and the phenylnaphthalene structure coupled with selective β-O-4 bond cleavage for synthesizing lignin-based epoxy resins with a controlled glass transition temperature
Abstract
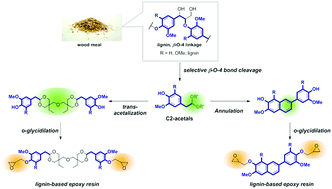
Lignin, the most abundant aromatic polymer in nature, is a potential renewable source for aromatic compounds in a post-oil era. Lignin is a prospective raw feedstock for epoxy resins because of its phenolic hydroxyl group, which offers a reaction point for glycidylation. However, precisely controlling the physical properties of a lignin-based epoxy resin based on the structure–property relationship is difficult because lignin is a heterogeneous macromolecule with unclarified substructures. The degradation of lignin into its monomers and their subsequent use as a building block for epoxy resins is another possible synthetic route, but this approach suffers from the formation of a wide variety of products by the chemical degradation of lignin. Herein, we successfully demonstrate that the selective degradation of lignin into the C2-acetal monomer by solvolysis in a hydrophobic solvent, i.e., toluene, containing methanolic H2SO4 and the subsequent synthesis of a cross-linked structure through the phenol units of C2-acetal enabled the rational control of the thermodynamic properties of a lignin-based epoxy resin. To introduce a flexible structure into the lignin-based epoxy resin, the transacetalization of C2-acetal with di(trimethylolpropane) (DTMP) was performed to obtain epoxy–DTMP–lignin. For comparison, we introduce a rigid structure by the annulation of C2-acetals to obtain epoxy-annulated lignin. The glass transition temperatures of epoxy–DTMP–lignin and epoxy-annulated lignin obtained by differential scanning calorimetry were 94 °C and 134 °C, respectively, demonstrating the achievement of the precise thermodynamic properties of lignin-based epoxy resins expected based on their structural design.

 Please wait while we load your content...
Please wait while we load your content...