Sustainable iron production from mineral iron carbonate and hydrogen
Abstract
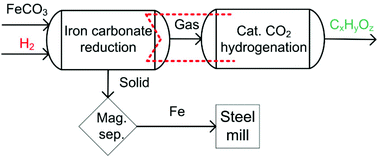
The reduction of iron ores with hydrogen is considered a promising CO2 breakthrough technology to mitigate CO2 emissions from the iron and steel industry. The state-of-the-art production of iron and steel from mineral iron carbonates (FeCO3) is based on the thermal decomposition of FeCO3 in air to produce hematite (Fe2O3) suitable for iron production. Our approach is to directly reduce FeCO3 with hydrogen to elemental iron, avoiding Fe2O3 formation. As a consequence, CO2 emissions can be decreased by 60% and up to 33% less reducing agent is needed for iron production. The development of environmentally benign production pathways needs to be based on a fundamental understanding of the reaction kinetics and mechanism. Therefore, thermogravimetry was used to determine the kinetics of the formation of iron from mineral iron carbonate and the concomitant decomposition of the accessory matrix carbonates of calcium, magnesium, and manganese. The isoconversional kinetic analysis according to the Ozawa–Flynn–Wall, Kissinger–Akahira–Sunose, and Friedman approach confirms the proposed parallel kinetic model. Multi-variate non-linear regression was used to determine the appropriate kinetic parameters. The conversion of iron carbonate to iron can be described with the two-dimensional Avrami-Erofeev model A2. Therefore, a temperature-controlled nucleation and diffusional growth mechanism is suggested for iron formation from mineral iron carbonate and hydrogen. The multi-parameter reaction models Cn-X and Bna can be used to describe the concomitant iron, calcium oxide, magnesium oxide, and manganese oxide formation without applying multi-step kinetics. The multi-parameter reaction models predict a conversion above 95% at 450 °C within less than 60 minutes reaction time. Unavoidably, 1 mole of carbon dioxide is always emitted when 1 mole of FeCO3 is converted into iron. Catalytic carbon dioxide hydrogenation (CCDH) can be applied to diminish inevitable CO2 emissions by chemical conversion into value-added carbon containing chemicals. Therefore, we propose a process that combines the improved iron production via direct FeCO3 reduction with CCDH as a follow-up reaction.


 Please wait while we load your content...
Please wait while we load your content...