Brønsted acid catalyzed transoximation reaction: synthesis of aldoximes and ketoximes without use of hydroxylamine salts†
Abstract
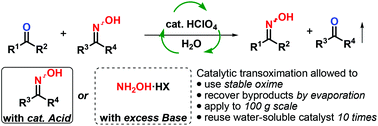
The transoximation reaction enables the transfer of an oxime to a carbonyl compound and is catalyzed by transoximase in the pupae of the silkworm. Inspired by this bio-synthetic pathway, we achieved the transoximation of oximes to aldehydes and ketones catalyzed by a Brønsted acid under mild conditions. Hydroxylamine salt, which necessitates a stoichiometric amount of base, was not required. NMR analysis clarified that this reaction proceeded through hydroxylamines generated by the successive hydrolysis of the oxime in situ. In addition, an environmentally benign method for catalytic transoximation was demonstrated in aqueous medium on a one hundred gram scale and the reaction filtrate containing the catalyst was recovered and reused over 10 times.


 Please wait while we load your content...
Please wait while we load your content...