Catalyst-free direct C–H trifluoromethylation of arenes in water–acetonitrile†
Abstract
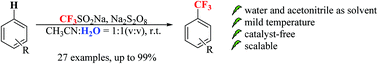
We describe a mild and practical strategy for the catalyst-free trifluoromethylation of arenes using the inexpensive solid sodium triflinate (Langlois’ reagent, NaSO2CF3) as a trifluoromethyl (CF3) source and solid sodium persulfate as an initiator. The reaction is environmentally friendly and proceeds at 30 °C in a water–acetonitrile mixture. This transformation can be easily scaled up to the gram level with excellent yield.

 Please wait while we load your content...
Please wait while we load your content...