Acid-catalysed carboxymethylation, methylation and dehydration of alcohols and phenols with dimethyl carbonate under mild conditions†
Abstract
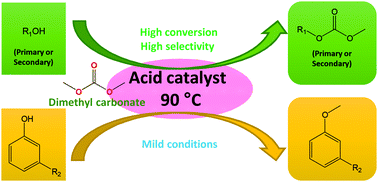
Dimethyl carbonate (DMC) chemistry has been extended to include acid-catalysed reactions of different aliphatic alcohols and phenols. For the first time, p-toluenesulfonic acid (PTSA), H2SO4, AlCl3 and FeCl3 have been shown to aid carboxymethylation for primary aliphatic alcohols at catalytic loadings with quantitative conversion and selectivity. For carboxymethylation of secondary alcohols, stoichiometric PTSA and catalytic AlCl3 both gave quantitative conversion and selectivity. Stoichiometric FeCl3 and H2SO4 promoted dehydration of linear aliphatic alcohols. Additionally FeCl3 catalysed methylation of cyclohexanol, whilst AlCl3 resulted in methylation of phenolic compounds. This research expands the range of potential application for DMC in green chemistry.

 Please wait while we load your content...
Please wait while we load your content...