Synthesis of phenols from hydroxymethylfurfural (HMF)†
Abstract
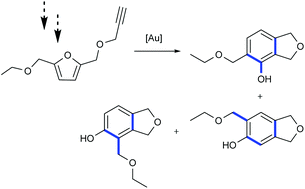
This study describes the use of hydroxymethylfurfural (HMF) as a precursor to phenols that are accessible within a few simple catalytic steps. A key step is an efficient transformation of HMF into its propargyl ether derivative under flow conditions. The latter was subsequently functionalised and used for gold-catalysed conversion into aromatic phenol derivatives. Special attention was paid on performing all of the chemical transformations under mild and environmentally benign conditions.

 Please wait while we load your content...
Please wait while we load your content...