Methyl vinyl glycolate as a diverse platform molecule†
Abstract
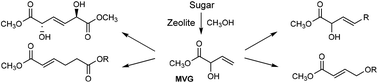
Methyl vinyl glycolate (methyl 2-hydroxybut-3-enoate, MVG) is available by zeolite catalyzed degradation of mono- and disaccharides and has the potential to become a renewable platform molecule for commercially relevant catalytic transformations. This is further illustrated here by the development of four reactions to afford industrially promising structures. Catalytic homo metathesis of MVG using Grubbs-type catalysts affords the crystalline dimer dimethyl (E)-2,5-dihydroxyhex-3-enedioate in excellent yield and with meso stereochemical configuration. Cross metathesis reactions between MVG and various long-chain terminal olefins give unsaturated α-hydroxy fatty acid methyl esters in good yields. [3,3]-Sigmatropic rearrangements of MVG also proceed in good yields to give unsaturated adipic acid derivatives. Finally, rearrangement of the allylic acetate of MVG proceeds in acceptable yield to afford methyl 4-acetoxycrotonate.

 Please wait while we load your content...
Please wait while we load your content...