Hydrogen peroxide/dimethyl carbonate: a green system for epoxidation of N-alkylimines and N-sulfonylimines. One-pot synthesis of N-alkyloxaziridines from N-alkylamines and (hetero)aromatic aldehydes†
Abstract
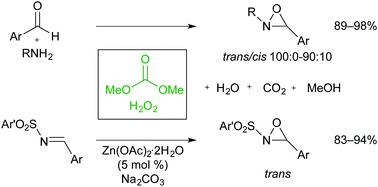
A green method for epoxidation of imines using an environmentally benign oxidant system, H2O2/dimethyl carbonate, was developed. N-Alkyloxaziridines were prepared in high yields from N-alkylamines and (hetero)aromatic aldehydes in one-pot fashion, whereas N-sulfonyloxaziridines have been prepared by using the same oxidant system and 5 mol% of Zn(OAc)2·2H2O as catalyst.


 Please wait while we load your content...
Please wait while we load your content...