Structure property relationships of biobased n-alkyl bisferulate epoxy resins†
Abstract
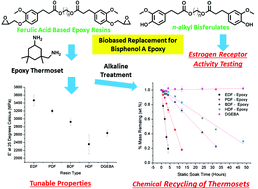
In this work, a series of bio-based chemically recyclable epoxy resins were synthesized from n-alkyl bisferulate esters that do not activate human estrogen receptor alpha (ERα). Viscosities of corresponding glycidyl ether n-alkyl bisferulate resins, determined by steady shear rheology, range from 12–9.4 Pa s. Activation energies of flow range from 83–96 kJ mol−1 and are similar to the diglycidyl ether bisphenol A (DGEBA). Thermomechanical properties of diglycidyl ether n-alkyl bisferulate resins cured with isophorone diamine were governed by the length of α,ω-diols that link glycidyl ether ferulate units. That is, the glassy phase modulus and alpha transition temperatures range from 3400–2400 MPa (at 25 °C) and 40–53 °C (peak of E′′), respectively. Furthermore, the onset of thermal degradation (Td5%) varied from 331–300 °C. Chemical recycling of cured epoxy resins was performed by static immersion in 10 w/w sodium hydroxide aqueous solutions at 60 °C. Times required for complete conversion of cured resins to water-soluble degradation products was also α,ω-diol length dependent and varied from 5 to 65 h. Thus, diglycidyl ether of n-alkyl bisferulate resins provides a viable biobased alternative to BPA epoxy resins as well as the option of chemical degradability and recovery of fillers in composite applications.

 Please wait while we load your content...
Please wait while we load your content...