Sunlight-promoted cyclization versus decarboxylation in the reaction of alkynoates with N-iodosuccinimide: easy access to 3-iodocoumarins†
Abstract
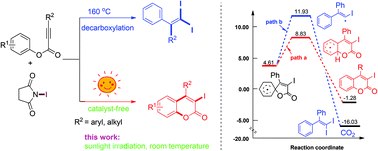
A sunlight-initiated radical conversion of aryl alkynoates to 3-iodocoumarins has been achieved using N-iodosuccinimide as the iodine source without the use of a catalyst or additive. Experimental, X-ray analysis, and computational studies indicate that the reaction under sunlight proceeds through iodination, spirocyclization and ring expansion to form the kinetic product. The sunlight-driven reaction provides a green pathway to especially valuable 3-halocoumarins derivatives.

 Please wait while we load your content...
Please wait while we load your content...