Selective catalytic Hofmann N-alkylation of poor nucleophilic amines and amides with catalytic amounts of alkyl halides†
Abstract
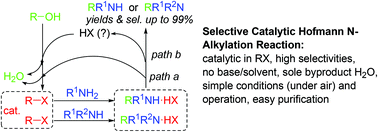
Using only catalytic amounts of alkyl halides in the reactions of poor nucleophilic amines/amides and alcohols led to a selective Hofmann N-alkylation reaction catalytic in alkyl halides, providing a practical and efficient method for the practical synthesis of mono- or di-alkylated amines/amides in high selectivities. This new method avoids the use of large amounts of bases, alkyl halides, and solvents, and generates water as the only byproduct. Preliminary mechanistic studies showed that alkyl halides are key intermediates/catalysts regeneratable in the reaction cycle.

 Please wait while we load your content...
Please wait while we load your content...