Formic acid as a sustainable and complementary reductant: an approach to fused benzimidazoles by molecular iodine-catalyzed reductive redox cyclization of o-nitro-t-anilines†
Abstract
Molecular iodine was found to be an excellent catalyst for reductive redox cyclization of o-nitro-t-anilines 1 into fused tricyclic or 1,2-disubtituted benzimidazoles 2. A range of functional groups such as halides (F, Cl, Br), methoxy, ester, trifluoromethyl, cyano, pyridine and even nitro groups were tolerated using formic acid as a clean, safe, user-friendly and complementary reductant. When iodine was used in a stoichiometric amount (50 mol%), the methodology allowed the direct synthesis of benzimidazole hydroiodides 2·HI in high yields by simple precipitation from the reaction mixture.
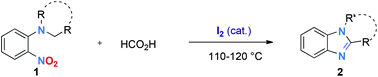

 Please wait while we load your content...
Please wait while we load your content...