Copper catalysed alkynylation of tertiary amines with CaC2via sp3 C–H activation†
Abstract
A mild and easy-to-handle protocol to produce propargylamines with a terminal alkyne through catalytic cross-coupling of tertiary amines and calcium carbide has been developed. The reaction proceeds via sp3 C–H bond activation and C–C coupling. Good to excellent yields were obtained for the corresponding propargylamines with both alkyl and aryl substitutions. The development of these functionalized propargylamines with a terminal alkyne group will offer a wider application for the synthesis of natural or pharmaceutical products due to their unique sp C–H reactivity.
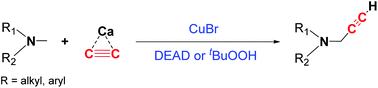

 Please wait while we load your content...
Please wait while we load your content...