Purification of indium by solvent extraction with undiluted ionic liquids†
Abstract
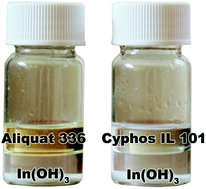
A sustainable solvent extraction process for purification of indium has been developed from a chloride aqueous feed solution using the ionic liquids Cyphos® IL 101 and Aliquat® 336. The high affinity of indium(III) for the ionic liquid phase gave extraction percentages above 95% over the HCl concentration range from 0.5 to 12 M. Attention was paid to the loading capacity of the ionic liquid phase and the kinetics of the extraction process. An extraction mechanism was proposed based on the relationship between the viscosity of the ionic liquid phase and the loading with indium(III) ions. Even for loadings as high as 100 g L−1, equilibrium was reached within 10 min. Due to the very high distribution ratio for indium(III), stripping of indium(III) from the ionic liquid phase was very difficult with water or acid solutions. However, indium could conveniently be recovered as In(OH)3 by precipitation stripping with a NaOH solution. Precipitation stripping has the advantage that no ionic liquid components are lost to the aqueous phase and that the ionic liquid is regenerated for direct re-use. The extraction of some metal ions that are commonly found as impurities in industrial indium process solutions, i.e. cadmium(II), copper(II), iron(III), manganese(II), nickel(II), tin(IV) and zinc(II), has been investigated. The distribution ratios for the different metal ions show that indium(III) can be purified efficiently by a combination of extraction, scrubbing and stripping stages. This new ionic liquid process avoids the use of volatile organic solvents.

 Please wait while we load your content...
Please wait while we load your content...