Stabilized Fe3O4 magnetic nanoparticles into nanopores of modified montmorillonite clay: a highly efficient catalyst for the Baeyer–Villiger oxidation under solvent free conditions†
Abstract
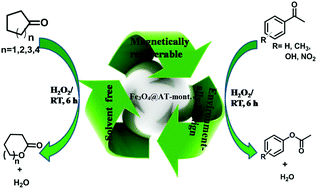
In situ generation of Fe3O4 magnetic nanoparticles (Fe3O4@AT-mont.) into the nanopores of modified montmorillonite (AT-mont.) clay has been carried out. Modification of the montmorillonite was done by acid (4 M HCl) activation under controlled conditions for generating nanopores, which act as a “host” for the Fe3O4 nanoparticles. The synthesized Fe3O4@AT-mont. was characterized by PXRD, TEM, SEM-EDX, XPS, VSM and surface area analysis. The average particle size of Fe3O4 nanoparticles was found to be around 10 nm and exhibit a face centered cubic (fcc) lattice geometry. Fe3O4@AT-mont. showed efficient catalytic activity for the Baeyer–Villiger oxidation of various cyclic and aromatic ketones in the presence of hydrogen peroxide as an oxidant at room temperature under solvent free conditions and exhibited conversion of up to 98%. The catalyst was magnetically recovered and recycled up to the third run without any significant loss of efficiency.


 Please wait while we load your content...
Please wait while we load your content...