Molecular structure, morphology and growth mechanisms and rates of 5-hydroxymethyl furfural (HMF) derived humins†
Abstract
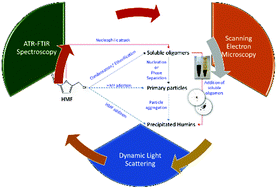
We apply ATR-FTIR spectroscopy, Scanning Electron Microscopy (SEM) and Dynamic Light Scattering (DLS) experiments to investigate the molecular structure, morphology and growth mechanism of 5-hydroxymethyl furfural (HMF) derived humins as a function of HMF conversion. Our FTIR data support a reaction pathway in which humins form either through a ring opening mechanism and/or through substitution at the α or β position via nucleophilic attack. The addition of DMSO as a co-solvent leads to significant changes in the FTIR spectra of humins. We find that the nucleophilic attack pathway is suppressed in the presence of DMSO co-solvent and rationalizes the very small humin particles (∼100 nm) observed in SEM images contrary to the large particles (with multimodal size distribution and largest particles of up to 3–4 μm) observed in neat water. DLS experiments under several reaction conditions further confirm the particle size distribution observed via SEM. A plausible reaction network for humin formation, which rationalizes qualitatively our experimental results as well as those reported in the literature, is also postulated.


 Please wait while we load your content...
Please wait while we load your content...