Mechanism and kinetics of tyrosinase inhibition by glycolic acid: a study using conventional spectroscopy methods and hydrogen/deuterium exchange coupling with mass spectrometry†
Abstract
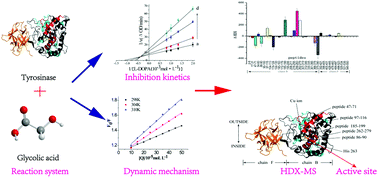
Tyrosinase is an enzyme that promotes enzymatic browning of fruits and vegetables, thereby reducing product quality. A variety of analytical tools were used to characterize the interactions between tyrosinase and a natural tyrosinase inhibitor (glycolic acid). Hydrogen/deuterium exchange coupling with mass spectrometry (HDX-MS) was used to elucidate the interaction mechanism between glycolic acid and tyrosinase. UV-visible, fluorescence and circular dichroism spectroscopy analysis indicated that glycolic acid inhibited tyrosinase activity in a mixed-type manner with an IC50 of 83 ± 14 μM. The results of these techniques suggested that glycolic acid bound to tyrosinase through hydrophobic attraction, and this interaction led to a pronounced conformational change of the enzyme molecules. HDX-MS analysis showed that the activity of tyrosinase was primarily inhibited by a structural perturbation of its active site (His 263). This study provides a comprehensive understanding of the interaction between glycolic acid and tyrosinase, which could lead to new approaches to control tyrosinase activity in foods and other products.
- This article is part of the themed collection: Food & Function 2017 Most Downloaded Articles


 Please wait while we load your content...
Please wait while we load your content...