Formation scheme and antioxidant activity of a novel Maillard pigment, pyrrolothiazolate, formed from cysteine and glucose
Abstract
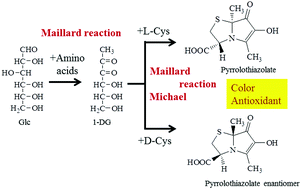
We recently identified 6-hydroxy-3[R],7a[S]-dimethyl-7-oxo-2,3-dihydropyrrolo[2,1-b]thiazole-3-calboxylic acid, a novel pyrrolothiazole derivative carrying a carboxy group and named pyrrolothiazolate, as a Mallard pigment formed from L-cysteine and D-glucose. Here we described the formation of its enantiomer, the plausible formation scheme of pyrrolothiazolate, and its antioxidant activity. When D-cysteine was used instead of L-cysteine in the reaction mixture, the enantiomer of pyrrolothiazolate was obtained. The carbon at position 1 of glucose was incorporated into two methyl groups of pyrrolothiazolate. The pigment was considered to be formed through 1-deoxyglucosone (1-DG). The dehydrated isomer of 1-DG would be condensed with the thiol and amino groups of cysteine. This condensate was dehydrated and cyclized to form pyrrolothiazolate. This compound was an antioxidant showing radical scavenging activity.
- This article is part of the themed collection: The Maillard reaction in food and nutrition

 Please wait while we load your content...
Please wait while we load your content...