Effect of chemical structure on the sonochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs)†
Abstract
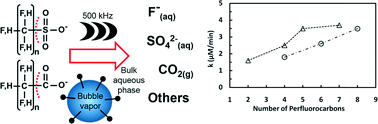
Perfluoroalkyl surfactants include chemicals characterized by a fully fluorinated carbon chain (hydrophobic and oleophobic tail) bound to a hydrophilic head (a carboxyl or sulfonic group). These compounds are toxic and highly resistant to chemical/biological attack, and some are known to be bio-accumulative. This study investigates the sonochemical degradation at 500 kHz of different carboxylic and sulfonic perfluoroalkyl and polyfluoroalkyl substances (PFASs, 1.7 mM total organic fluorine) to assess the effect of chain length, functional head group, and substituents (–CH2–CH2– moiety and ether group) on the degradation rate. Under these conditions, the rates of defluorination determined for two widely used perfluoroalkyl substances, perfluorooctanesulfonate (PFOS) and perfluorooctanoic acid (PFOA), were 3.5 to 3.7 μM F− min−1, respectively. The degradation rate of perfluoroalkyl sulfonates decreased with the perfluorocarbon chain length as indicated by the 1.3 and 1.9-fold lower defluorination rates for perfluorohexane- and perfluorobutane sulfonate than that of PFOS. A similar trend was observed during the sonolysis of perfluoroalkyl carboxylate analogs with 6, 5 or 3 carbon atoms which had 1.1-, 1.8-, and 2.3-fold lower defluorination rates, respectively, than that of PFOA. Furthermore, perfluoroalkyl compounds appeared more amenable to sonolysis than the polyfluoroalkyl analogues with the same number of C atoms (defluorination rate of PFOS/6 : 2 fluorotelomer sulfonate ≈ 2.3). The results demonstrate that sonolysis is a promising approach to treat PFASs in aqueous streams. Furthermore, they underscore that the chemical structure of PFASs has a marked effect on the rate at which they undergo sonochemical degradation.

 Please wait while we load your content...
Please wait while we load your content...