Leveraging the new predictive toxicology paradigm: alternative testing strategies in regulatory decision-making†
Abstract
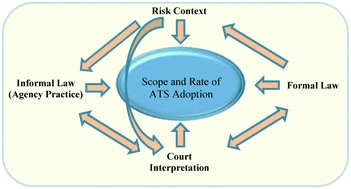
Although toxicity data is critical to effective risk prevention and management, comprehensive health and safety data is not available for the vast majority of chemicals in use today. Rapid development of new engineered nanomaterials exacerbates the dilemma even further. Emerging alternative testing approaches offer a solution to this dilemma. Traditional toxicity testing predicts human disease based upon its occurrence in other species such as rodents and rabbits. Alternative testing strategies (ATS) seek to reduce, refine or replace the use of animals, minimize cost and diminish uncertainty by placing greater reliance upon mechanistically-based in vitro and in silico methods. While significant advances have recently been made in the science of alternative testing, little of that science has worked its way into regulatory actions by EPA. Recent reforms to the federal Toxic Substances Control Act include provisions meant to advance regulatory use of ATS. This article asks whether the legislation will make any appreciable difference in the adoption of ATS for regulatory purposes. Recognizing that the scope and speed of adoption will depend on the specific legal-institutional environment in question, we present and apply a conceptual model that takes that environment into account in the context of the federal Environmental Protection Agency's (EPA) regulation of chemicals. We use that model to explore EPA's historical usage of alternative testing strategies, identifying certain features of the legal-institutional environment that influenced EPA usage. We then turn to the reform legislation and consider whether it is likely to alter the relevant features of the legal-institutional environment.
- This article is part of the themed collection: Sustainable Nanotechnology Organization 2015

 Please wait while we load your content...
Please wait while we load your content...