Formation of trihalomethanes and haloacetic acids during chlorination of functionalized carbon nanotubes†
Abstract
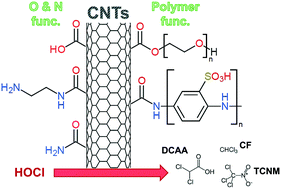
We have previously shown that nitrogen-functionalized carbon nanotubes (CNTs) represent both a precursor and source of N-nitrosodimethylamine (NDMA), a nitrogenous disinfection byproduct (DBP). Here, we explore the potential for non-functionalized, oxidized (via strong acid, ozone or chlorine), and polymer-functionalized CNTs to serve as precursors for chlorinated DBPs including regulated trihalomethanes (THMs; e.g., chloroform) and chlorinated haloacetic acids (or Cl-HAAs; e.g., mono-, di- and trichloroacetic acid). During chlorination experiments (typically 10 mg L−1 CNTs, 15 mg L−1 HOCl, pH 8), formation of chloroform (CF) and Cl-HAAs was greatest for CNTs functionalized with the polymers PABS and PEG, producing as much as 3.5 μg of CF per mg-CNT and 10.2 μg of Cl-HAAs per mg-CNT after 4 h of chlorination (with yields increasing over time). Further, while CF and Cl-HAAs production in PABS-CNT systems was comparable to that generated by the free polymer (i.e., PABS in the absence of CNTs), their formation was unique for PEGylated CNTs (i.e., CF and Cl-HAAs were not observed during chlorination of PEG alone, nor for physical mixtures of CNTs and PEG). While non-functionalized and acid-oxidized CNTs did not produce any measurable CF or Cl-HAAs despite exerting a chlorine demand, the ability of CNTs to function as precursors for these DBPs increased with their extent of oxidation via ozone and chlorine. For CNTs oxidized via ozonation or chlorination we observed a strong, linear correlation between formation of CF and Cl-HAAs (primarily trichloroacetic acid) and CNT surface oxygen concentration from X-ray photoelectron spectroscopy. Aside from CF and Cl-HAAs, chloropicrin (or nitrochloroform), a potent toxin used as a broad-spectrum pesticide, was also identified as a major DBP generated during chlorination of nitric acid functionalized CNTs, producing ∼1 μg per mg-CNT after 4 h. We propose its formation is the result of residual forms of oxidized nitrogen that persist on CNT surfaces after nitric acid oxidation. Although measured yields (along with low CNT levels expected in water/wastewater treatment) suggest DBP production from CNTs will be limited compared to traditional precursors (e.g., organic matter), some proposed CNT applications that utilize higher mass loadings (e.g., CNT-based filters) may produce appreciable DBP levels when in contact with residual chlorine.

 Please wait while we load your content...
Please wait while we load your content...