Transformation kinetics of silver nanoparticles and silver ions in aquatic environments revealed by double stable isotope labeling†
Abstract
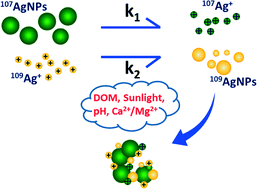
Silver nanoparticles (AgNPs) are rather mutable in water columns, and the oxidation of AgNPs to release Ag+ and reduction of Ag+ to regenerate AgNPs exist simultaneously in certain environments, making it rather difficult to monitor the reaction kinetics. In this study, we synthesized isotopically labeled AgNPs (99.5% 107Ag, 107AgNPs) and AgNO3 (99.81% 109Ag, 109AgNO3). For the first time, two stable Ag isotopes were used in the same experiment to track the transformation kinetics of AgNPs and Ag+ independently in aquatic environments. It was found that the oxidation of AgNPs dominated the reaction in simple water solutions containing both 107AgNPs and 109Ag+. Sunlight significantly accelerated the dissolution of the 107AgNPs, but longer solar irradiation (8 h) triggered aggregation of the 107AgNPs and therefore reduced the reaction rate. With the addition of 5 mg C L−1 dissolved organic matter, the reduction of 109Ag+ played the leading role. The corrected concentration of dissolved 107Ag+ began to decrease after some time, indicating other reduction mechanisms were happening. An elevated pH (pH 8.5) could even completely inhibit the oxidation of 107AgNPs. All the reactions seemed stalled at low temperature (6 °C) except the dissolution of 107AgNPs under solar irradiation, suggesting a non-negligible effect of sunlight. The presence of divalent cations induced agglomeration of 107AgNPs, but the reduction of 109Ag+ was not significantly affected. These findings implied that the transformation between AgNPs and Ag+ was rather complex and greatly depended on the external conditions. Given the fact that Ag+ has been shown to be much more toxic than AgNPs, the speciation change may dramatically impact the final toxicity and bioavailability of AgNPs, so there is a high demand for assessing the environmental risks of AgNPs under more realistic conditions.

 Please wait while we load your content...
Please wait while we load your content...