Effects of low-molecular-weight organic acids on the dissolution of hydroxyapatite nanoparticles†
Abstract
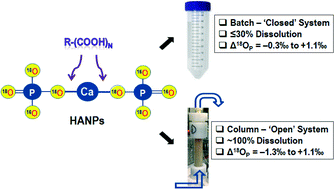
Hydroxyapatite nanoparticles (HANPs) have recently been advocated as a highly efficient and environmentally benign ‘green’ phosphorus (P) nanofertilizer in modern agriculture. However, knowledge of how low-molecular-weight organic acids (LMWOAs) secreted by plants in agricultural soils mediate the dissolution of HANPs and release of dissolved phosphate is nonexistent. Here, three of the most commonly occurring LMWOAs (acetic, oxalic, and citric acids) were evaluated for their roles in HANPs dissolution in both batch and column systems. Particularly, O-isotopic ratios of dissolved phosphate during HANPs dissolution were measured to disentangle mechanisms controlling isotope fractionation. Our results reveal that in a batch system HANPs dissolution was very fast but the overall dissolution efficiency was low (≤30%), unlike in a column system where ∼100% dissolution was achieved. The low dissolution efficiency of HANPs in the batch system was due predominantly to rapid consumption of protons, whereas in the column system the HANPs were progressively dissolved by low pH LMWOAs and reaction products were eluted out. Regardless of LMWOA type and experimental system, the isotopically lighter phosphate (P16O4) was preferentially released during the initial phases of dissolution and dissolved phosphate became gradually P18O4-enriched with time. This fractionation was less in batch (−0.3‰ to +1.1‰) than in column (−1.3‰ to +1.1‰) systems due primarily to lower dissolution efficiency and higher P16O4 and P18O4 exchange between HANPs and dissolved phosphates. The Rayleigh model well described O-isotopic fractionation of dissolved phosphate under different LMWOAs. Overall, our findings provide important insights into the dissolution kinetics and O-isotopic evolution of phosphate-based NPs that are relevant to plant–soil systems particularly at the rhizosphere.

 Please wait while we load your content...
Please wait while we load your content...