Sulfate formation catalyzed by coal fly ash, mineral dust and iron(iii) oxide: variable influence of temperature and light†
Abstract
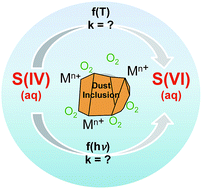
Recent atmospheric field and modeling studies have highlighted a lack of understanding of the processes responsible for high levels of sulfate aerosol in the atmosphere, ultimately arising from a dearth of experimental data on such processes. Here we investigated the effect of temperature and simulated solar radiation on the catalytic oxidation of S(IV) to S(VI) (i.e., sulfite to sulfate) in aqueous suspensions of several metal-containing, atmospherically relevant particles including coal fly ash (FA), Arizona test dust (ATD) and an iron oxide (γ-Fe2O3). The effect of temperature and light on S(IV) oxidation was found to be very different for these three samples. For example, in the presence of FA and γ-Fe2O3 the temporal evolution of dissolved Fe(II) (formed via reductive particle dissolution) correlated with S(IV) oxidation. Accordingly, we propose that S(IV) oxidation in most of these systems initially occurs primarily at the particle surface (i.e., a heterogeneous reaction pathway), although a solution-phase (i.e., homogeneous) catalytic pathway also contributes over later timescales due to the formation and accumulation of dissolved Fe(III) (generated via oxidation of dissolved Fe(II) by O2). It is likely that the homogeneous reaction pathway is operative at initial times in the presence of γ-Fe2O3 at 25 °C. In contrast, S(IV) oxidation in the presence of ATD appears to proceed entirely via a heterogeneous reaction, which notably does not lead to any iron dissolution. In fact, the greater overall rate of S(IV) loss in the presence of ATD compared to FA and γ-Fe2O3 suggests that other factors, including greater adsorption of sulfite, transition metal ion (TMI) catalysis by other metal ions (e.g., Ti), or different species of iron in ATD, play a role. Overall these studies suggest that the rate, extent and products of atmospheric S(IV) oxidation can be highly variable and dependent upon the nature of aerosol sources and ambient conditions (e.g., temperature and irradiance). Ultimately, such complexity precludes simple, broadly generalized schemes for this reaction when modeling atmospheric processes involving diverse components of different mineral dust aerosol as well as other metal-containing aerosol.

 Please wait while we load your content...
Please wait while we load your content...