A bifunctional solid state catalyst with enhanced cycling stability for Na and Li–O2 cells: revealing the role of solid state catalysts†
Abstract
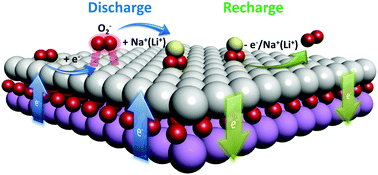
Solid state catalysts play a critical role in peroxide alkali metal–O2 cells. However, the underlying mechanism behind the catalytic activity remains controversial due to the different nature of oxygen reduction and evolutions reactions (ORR, OER) in non-aqueous cells compared to those in classic aqueous based reactions. In the present study, we reveal a detailed spectroscopic and electrochemical picture of the mechanism of catalytic activity in Na– and Li–O2 cells. We demonstrate that ORR and OER catalytic activity in alkali metal–O2 cells primarily originates from the stabilization of O2− intermediates on the catalyst surface during the electrochemical reaction. Monitoring the electronic state of the solid state catalyst during the ORR and OER revealed a dynamic interaction occurring between the catalyst and the discharge product. The morphology and composition of discharge products is also illustrated to be influenced by solid state catalysts. The findings of the present study suggest that catalysts with a higher oxygen-bonding capability may exhibit a higher catalytic activity in alkali metal–O2 cells.


 Please wait while we load your content...
Please wait while we load your content...