A layered Na1−xNiyFe1−yO2 double oxide oxygen evolution reaction electrocatalyst for highly efficient water-splitting†
Abstract
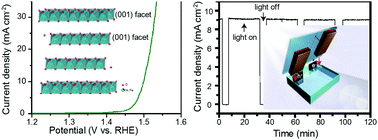
Transition metal Ni- and Co-based oxides are potential candidates to replace expensive and scarce noble metal-based oxygen evolution reaction (OER) catalysts such as IrO2 and RuO2, which are required for efficient hydrogen production from solar water splitting and rechargeable energy storage technologies. So far, layered NiFe double hydroxide represents the best OER activity among all Ni- and Co-based oxides. Here, we report new layered Na1−xNiyFe1−yO2 double oxide OER catalysts exhibiting activity and stability surpassing those of noble metal OER catalysts including IrO2 and RuO2, and a layered NiFe double hydroxide OER catalyst. The superior catalytic properties can be ascribed to the layered structure as well as the enhanced covalency of Ni and Fe. Powered by a lead halide perovskite solar cell with a power conversion efficiency of 14.69%, a two-electrode solar water-splitting device combining a Na0.08Ni0.9Fe0.1O2 OER catalyst with a NiP hydrogen evolution reaction catalyst delivers a solar-to-hydrogen conversion efficiency of 11.22%. Our design and fabrication strategies offer insights for developing highly active electrocatalysts for water splitting and metal–air batteries.


 Please wait while we load your content...
Please wait while we load your content...