In situ investigation of the formation and metastability of formamidinium lead tri-iodide perovskite solar cells†
Abstract
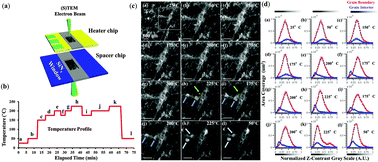
Organic–inorganic perovskites have emerged as an important class of next generation solar cells due to their remarkably low cost, band gap, and sub-900 nm absorption onset. Here, we show a series of in situ observations inside electron microscopes and X-ray diffractometers under device-relevant synthesis conditions focused on revealing the crystallization process of the formamidinium lead-triiodide perovskite at the optimum temperature of 175 °C. Direct in situ observations of the structure and chemistry over relevant spatial, temporal, and temperature scales enabled identification of key perovskite formation and degradation mechanisms related to grain evolution and interface chemistry. The lead composition was observed to fluctuate at grain boundaries, indicating a mobile lead-containing species, a process found to be partially reversible at a key temperature of 175 °C. Using low energy electron microscopy and valence electron energy loss spectroscopy, lead is found to be bonded in the grain interior with iodine in a tetrahedral configuration. At the grain boundaries, the binding energy associated with lead is consequently shifted by nearly 2 eV and a doublet peak is resolved due presumably to a greater degree of hybridization and the potential for several different bonding configurations. At the grain boundaries there is adsorption of hydrogen and OH− ions as a result of residual water vapor trapped as a non-crystalline material during formation. Insights into the relevant formation and decomposition reactions of formamidinium lead iodide at low to high temperatures, observed metastabilities, and relationship with the photovoltaic performance were obtained and used to optimize device processing resulting in conversion efficiencies of up to 17.09% within the stability period of the devices.

 Please wait while we load your content...
Please wait while we load your content...