Multidentate 2-pyridyl-phosphine ligands – towards ligand tuning and chirality†
Abstract
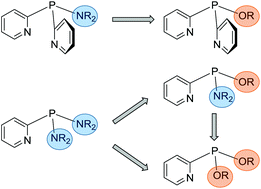
In the current work a range of multidentate pyridyl-phosphine ligands are synthesised with tuneable electronic and steric character, through the incorporation of a variety of alcohols into (amino)pyridyl-phosphine frameworks. The stoichiometric reactions of compounds of the type (R2N)xP(2-py)3−x (2-py = 2-pyridyl) with alkyl as well as aryl alcohols result in the formation of (alkoxy)pyridyl-phosphines (RO)xP(2-py)3−x (R = Me, 2-Bu, Ph). This synthetic procedure also allows the introduction of enantiomerically pure alcohols, like (R)-(−)-2-BuOH and (S)-(+)-2-BuOH, and as such provides a very convenient two-step route to chiral multidentate pyridyl-phosphine ligand sets. Using the bis-amino-phosphine (Et2N)2P(2-py), the stepwise introduction of alcohols enables the synthesis of racemic alkoxy-amino-phosphines (R2N)(RO)P(2-py), as well as alkoxy-phosphines (RO)2P(2-py) and therefore offers easy access to a library of different pyridyl-phosphine ligands. Coordination studies of the (amino)pyridyl-phosphines and (alkoxy)pyridyl-phosphines with copper(I) reveal that ligands with two N donor atoms form dimeric arrangements, while (PhO)2P(2-py), in-corporating only one N donor atom, shows completely different coordination behaviour.


 Please wait while we load your content...
Please wait while we load your content...