Structures and magnetic properties of dysprosium complexes: the effect of crystallization temperature†
Abstract
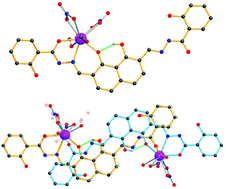
Two new dysprosium complexes, [Dy(H5L)(NO3)2(CH3OH)2]·4CH3OH (1) and [Dy2(H5L)2(NO3)4(H2O)2]·10CH3OH (2), were isolated from the reaction of a novel 1,8-naphthalenediol-based ligand N,N′-((1,8-dihydroxynaphthalene-2,7-diyl)bis(methanylylidene))bis(2-hydroxybenzohydrazide), H6L with dysprosium(III) nitrate upon crystallization at different temperatures. Because of a low-symmetrical coordination environment, both complexes display only field-induced single molecular magnetic (SMM) behavior. Interestingly, complex 2 containing two dysprosium ions shows field-induced multiple relaxation processes, whereas only one relaxation process is observed for complex 1. The remarkably different behavior observed in 2 is mainly ascribed to the weak intra- or intermolecular interactions between the two DyIII centres in this complex.


 Please wait while we load your content...
Please wait while we load your content...