Structure investigations of group 13 organometallic carboxylates†
Abstract
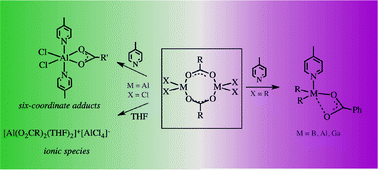
The octet-compliant group 13 organometallics with highly polarized bonds in the metal coordination sphere exhibit a significant tendency to maximize their coordination number through the formation of adducts with a wide range of neutral donor ligands or by self-association to give aggregates containing tetrahedral and higher coordinated aluminium centres, and even in some cases molecular complexes equilibrate with ionic species of different coordination numbers of the metal centre. This work provides a comprehensive overview of the structural chemistry landscape of the group 13 carboxylates. Aside from a more systematic approach to the general structural chemistry of the title compounds, the structure investigations of [R2M(μ-O2CPh)]2-type benzoate complexes (where M = B, Al and Ga) and their Lewis acid–base adducts [(R2M)(μ-O2CPh)(py-Me)] are reported. DFT calculations were also performed to obtain a more in-depth understanding of both the changes in the bonding of group 13 organometallic carboxylate adducts with a pyridine ligand.

 Please wait while we load your content...
Please wait while we load your content...