A pentacoordinated norbornenyl-acyl-rhodium(iii) complex as a likely intermediate in the catalytic hydroacylation of norbornadiene†
Abstract
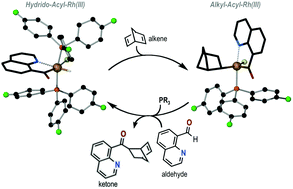
[RhCl(NCO)(nbyl)(PR3)] (nbyl = σ-norbornenyl; NCO = quinoline-8-acyl; R = p-F-C6H4) (1) has been synthesized by the reaction of [Rh(nbd)Cl]2 (nbd = norbornadiene) with 2 equivalents of NCHO (quinoline-8-carbaldehyde) and 2 equivalents of PR3. Compound 1 has been fully characterized in solution and also in the solid state by X-ray diffraction. Compound 1 shows low stability in solution and undergoes slow ring closure isomerization to [RhCl(NCO)(ntyl)(PR3)] (ntyl = σ-nortricyclyl) (2) after 12 hours. Reaction of 1 with an extra equivalent of aldehyde (NCHO) and PR3 led to the formation of [RhCl(H)(NCO)(PR3)2] (3) and an equivalent of ketone, which is a hydroacylation product. The catalytic activity of 3 in the hydroacylation of nbd with NCHO is reported as well as the catalytic activity of compound 1. Compounds 1 and 3 are proposed as intermediate species in the catalytic hydroacylation of norbornadiene with NCHO.


 Please wait while we load your content...
Please wait while we load your content...