Copper-based energetic MOFs with 3-nitro-1H-1,2,4-triazole: solvent-dependent syntheses, structures and energetic performances†
Abstract
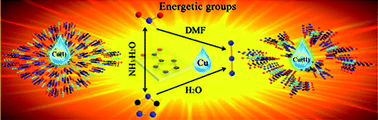
The persistent challenge in the field of energetic materials is how to synthesize energetic compounds with high density, high heat of detonation and outstanding detonation performance by gathering the maximum number of energetic groups in the smallest volume. The self-assembly of energetic groups with metal ions is crucially influenced by the solvent conditions. Here, the reaction of Cu(NO3)2·3H2O with 3-nitro-1H-1,2,4-triazole (Hntz) in aqueous ammonia under hydrothermal conditions via a self-assembly strategy yielded the Cu(I) energetic compound [Cu(ntz)]n (1). In order to further enhance the energetic property, an N3− anion was introduced into the system and two Cu(II) energetic compounds, [Cu(ntz)(N3)(DMF)]n (2) and [Cu(ntz)(N3)(H2O)]n (3), were successfully synthesized under different solvent conditions. Structural analyses show that compound 1 features a compacted 3D structure framework and compounds 2–3 exhibit 1D butterfly-like chain structures. The experimental results reveal that 1 possesses attractive thermal stability up to 315.0 °C and 1–3 present excellent insensitivity. Importantly, the heat of detonation of compound 2 has been factually improved due to the abundant energetic bonds in the coordinated DMF molecules compared to 1 and lots of energies are taken away during the release of the coordinated solvent molecules in the low temperature range resulting in the obvious decreases in detonation pressure and detonation velocity for compounds 2–3, which further exemplifies that the subtle change of reaction conditions may have a crucial effect on the resultant detonation performance. In addition, the detonation performances of 1–3 calculated by both a simple method for metal-containing explosives developed by Pang et al. and the commercial program EXPLO5 v6.01, are discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...