Inter- and intra-molecular C–H borylation for the formation of PAHs containing triarylborane and indole units†
Abstract
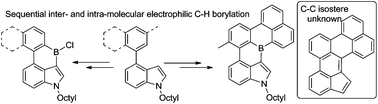
Inter-/intra-molecular electrophilic C–H borylation of C4-substituted indoles enables the formation of fused polycyclic aromatic structures containing triarylborane and N-heterocyclic units. These compounds are B–(C)n–N isosteres of carbocyclic PAHs that do not contain B–N bonds and comparison of one pair of BN/CC isosteres reveals that different resonance structures dominate. These compounds are highly sensitive to protodeboronation, of both the chloroborane intermediates and the mesityl protected products, which results in low isolated yields of the latter. Protodeboronation can be utilised productively for a C–H directed, C–H electrophilic borylation to make a previously unknown pinacol boronate ester by selective protodeboronation of the chloroborane intermediate. Intermolecular and double intramolecular electrophilic C–H borylation of a C4-substituted indole leads to a more highly fused structure containing two boracycles which represents a B–(C)n–N analogue of the unknown carbon isostere indeno[1,7ab]perylene.


 Please wait while we load your content...
Please wait while we load your content...