Thermodynamics of biphasic lanthanide extraction by tripodal diglycolamide: a solution calorimetry study
Abstract
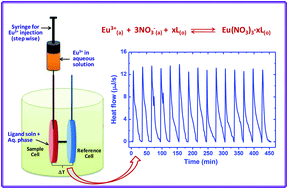
Isothermal titration calorimetry was employed for the direct measurement of the enthalpy of extraction (ΔHextr) of Eu(NO3)3 by using a tripodal diglycolamide (T-DGA) ligand dissolved in n-dodecane containing 5% (v/v) 2-decanol. The enthalpy of extraction obtained by titration calorimetry was in good agreement with the enthalpy of extraction calculated from the temperature dependence of the distribution coefficients by using the van't Hoff equation. The Gibbs free energy and the entropy of extraction (ΔGextr and ΔSextr) for the extraction of Eu(NO3)3 by T-DGA were also obtained by solvent extraction experiments. The complexation of Eu3+ with T-DGA in a mixture of acetonitrile/nitric acid was also studied by spectrophotometry and calorimetry to determine the stability constants and the enthalpy of complexation (ΔHcomp) for the Eu3+/T-DGA complexes in a single phase. The enthalpy of complexation, though obtained in a solvent different from that in the solvent extraction, allows a rough estimate of the enthalpy of phase transfer of the Eu3+/T-DGA complexes from the aqueous phase to the organic phase.

 Please wait while we load your content...
Please wait while we load your content...