Terminal solvent effects on the anisotropy barriers of Dy2 systems†
Abstract
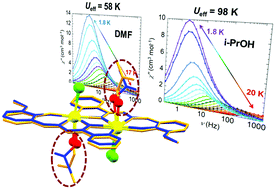
A family of three dinuclear dysprosium complexes have been successfully synthesized and studied in terms of their magnetic properties. Complexes 1 and 2 share the formula [Dy2(ovph)2Cl2(solvent)2], where H2ovph = pyridine-2-carboxylic acid [(2-hydroxy-3-methoxyphenyl)methylene] hydrazide, and solvent = DMF (1), i-PrOH (2), while complex 3, [Dy2(ovph)2Cl2(H2O)3(EtOH)], exhibits differences in terms of the identity and number of coordinated solvent molecules. Thus, we investigate the impact of terminally bonded solvent molecules on the slow relaxation dynamics of {Dy2} SMMs, a parameter which can sometimes be overlooked in the quest to attain higher energy barriers. Notably, the exchange of DMF for i-PrOH, both of which coordinate through a single oxygen atom, results in a near 2-fold increase in Ueff, from 58 to 98 K, for 1 and 2, respectively.
- This article is part of the themed collection: Molecular Spintronics : The role of Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...