Platinum(0)-mediated C–O bond activation of ethers via an SN2 mechanism†
Abstract
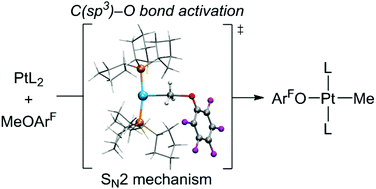
A computational study of the C(methyl)–O bond activation of fluorinated aryl methyl ethers by a platinum(0) complex Pt(PCyp3)2 (Cyp = cyclopentyl) (N. A. Jasim, R. N. Perutz, B. Procacci and A. C. Whitwood, Chem. Commun., 2014, 50, 3914) demonstrates that the reaction proceeds via an SN2 mechanism. Nucleophilic attack of Pt(0) generates an ion pair consisting of a T-shaped platinum cation with an agostic interaction with a cyclopentyl group and a fluoroaryloxy anion. This ion-pair is converted to a 4-coordinate Pt(II) product trans-[PtMe(OArF)(PCyp3)2]. Structure-reactivity correlations are fully consistent with this mechanism. The Gibbs energy of activation is calculated to be substantially higher for aryl methyl ethers without fluorine substituents and higher still for alkyl methyl ethers. These conclusions are in accord with the experimental results. Further support was obtained in an experimental study of the reaction of Pt(PCy3)2 with 2,3,5,6-tetrafluoro-4-allyloxypyridine yielding the salt of the Pt(η3-allyl) cation and the tetrafluoropyridinolate anion [Pt(PCy3)2(η3-allyl)][OC5NF4]. The calculated activation energy for this reaction is significantly lower than that for fluorinated aryl methyl ethers.


 Please wait while we load your content...
Please wait while we load your content...