The effects of substituent position on kinetics of benzene vapour adsorption onto 3-phenylphenoxy substituted metal-free and metallo-phthalocyanines thin films†
Abstract
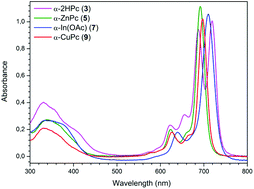
The preparation of metal-free, Zn(II), In(III), and Cu(II)-phthalocyanines containing tetrakis-(3-phenylphenoxy) groups was achieved by employing 3-(3-phenylphenoxy)phthalonitrile (1) and 4-(3-phenylphenoxy)phthalonitrile (2) as starting materials. The phthalonitriles and phthalocyanines were characterized by elemental analysis, infrared, proton nuclear magnetic resonance, ultraviolet-visible, and matrix-assisted laser desorption/ionization time-of-flight mass spectroscopic techniques. The effect of the substituent group on the kinetics of benzene vapour adsorption onto these novel compounds was examined using three kinetics models: the pseudo first-order model, the Elovich equation, and a simple adsorption–desorption model. Results show that the benzene adsorption kinetics strongly depend on the position of the substituent groups.

 Please wait while we load your content...
Please wait while we load your content...