The separation mechanism of Am(iii) from Eu(iii) by diglycolamide and nitrilotriacetamide extraction reagents using DFT calculations†
Abstract
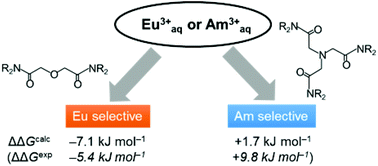
Relativistic density functional calculations were applied to study the separation behaviors of the Am(III) ion from the Eu(III) ion by diglycolamide (DGA) and nitrilotriacetamide (NTA) ligands in order to understand the difference in the separation mechanism of their reagents. The complexation reaction was modeled on the basis of previous experimental studies. The calculated energies based on stabilization by complex formation at the ZORA-B2PLYP/SARC level predicted that the DGA reagent preferably coordinated to the Eu(III) ion when compared with the Am(III) ion. In contrast, the NTA reagent selectively coordinated to the Am(III) ion when compared with the Eu(III) ion. These results reproduced the experimental selectivity of DGA and NTA ligands toward Eu(III) and Am(III) ions. Mulliken's population analyses implied that the difference in the contribution of the bonding property between the f-orbital of Am and donor atoms determined the comparative stability of Eu and Am complexes.


 Please wait while we load your content...
Please wait while we load your content...