Dehydrogenation of benzyl alcohol with N2O as the hydrogen acceptor catalyzed by the rhodium(i) carbene complex: insights from quantum chemistry calculations†
Abstract
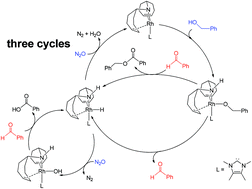
The detailed mechanisms of the dehydrogenation of benzyl alcohol with N2O as the hydrogen acceptor catalyzed by the rhodium(I) carbene complex for the formation of the corresponding carboxylic acid or ester have been investigated via density functional theory (DFT) calculations at the M06 level of theory. Three cycles were considered for the formation of benzaldehyde, benzyl benzoate and benzoic acid. On the basis of the calculations, the rate-determining step for these three cycles is involved in N2O activation by the rhodium ammine hydride complex with an activation barrier of only 22.6 kcal mol−1, which is different from the previous mechanism proposed by Gianetti and co-workers, where the hydride is transferred from the Rh atom to the oxygen atom of N2O with a barrier of 30.5 kcal mol−1. In addition, the calculations also demonstrated that one more N2O is necessary to give benzoic acid, and the reaction can only take place under anhydrous conditions. Present calculations are in good agreement with the experimental observations and provide new insights into the dehydrogenation of benzyl alcohol with N2O as the hydrogen acceptor.

 Please wait while we load your content...
Please wait while we load your content...