A comparative study on the CO2 hydrogenation catalyzed by Ru dihydride complexes: (PMe3)4RuH2 and (Me2PCH2CH2PMe2)2RuH2†
Abstract
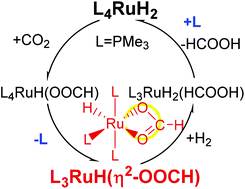
The phosphine complexes of Ru dihydride are model catalysts for CO2 hydrogenation. Despite many theoretical studies, important questions remain unresolved regarding the underlying catalytic mechanisms. We report a comparative study by using density functional theory on two catalysts, (PMe3)4RuH2 and (dmpe)2RuH2, with dmpe = Me2PCH2CH2PMe2, for which very different mechanisms have been suggested in previous studies. By comparing their energy profiles along all possible reaction paths side by side, we are able to clarify the similarity and difference between them, and provide a consistent account for all the experimental observations reported, including the kinetic models, the cis to trans transformation of (dmpe)2RuH2, and the significant enhancement of the catalytic rate in supercritical CO2 for (PMe3)4RuH2 and its lack thereof for (dmpe)2RuH2. The crucial difference between the two mechanisms involves the formation of an intermediate, in which a formate ion binds to Ru as a bidentate ligand. When this step results in the dissociation of a ligand, the reaction rate is enhanced under supercritical conditions, due to the increase in entropy, which should be a valid consideration for other catalytic reactions as well.

 Please wait while we load your content...
Please wait while we load your content...