Benzimidazole–triazole ligands with pendent triazole functionality: unexpected formation and effects on copper-catalyzed aerobic alcohol oxidation†
Abstract
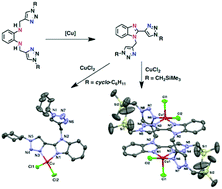
A series of benzimidazole–triazole ligands (NN′) having a pendent triazole arm with different triazole substituents including CH2Ph (3a), cyclo-C6H11 (3b), and CH2SiMe3 (3c) were obtained in moderate yields from Cu-catalyzed oxidative C–N cyclization of the respective amine-triazole compounds N,N′-bis((1-R-1,2,3-triazol-4-yl)methyl)benzene-1,2-diamine (2a–2c). Treatment of CuCl2 with one equiv. of the benzimidazole–triazole ligands afforded the corresponding CuII complexes with the empirical formula of Cu(NN′)Cl2 (4a–4c). Crystal structures of 4b and 4c reveal mononuclear and dinuclear CuII complexes, respectively. Despite the differences in triazole substituents and their solid state structures, ESR spectra indicate the same molecular structures in CH3CN solution whereas CV data suggest similar redox potentials for 4a–4c. Catalytic activities for aerobic oxidation of benzyl alcohol to benzaldehyde follow this trend: 4c > 4a > 4b. In addition, the catalytic system 4c/TEMPO/Cu0/NMI (TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxyl, NMI = N-methylimidazole) exhibited high activities for oxidation of activated alcohols (i.e., benzyl alcohol derivatives and allylic alcohol) in CH3CN at room temperature.


 Please wait while we load your content...
Please wait while we load your content...