A unified set of experimental organometallic data used to evaluate modern theoretical methods†
Abstract
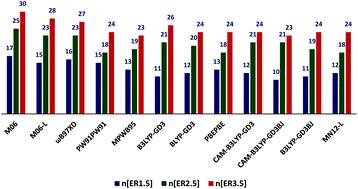
We applied a test set of ligand dissociation enthalpies derived entirely from a unified experimental approach to evaluate the efficacy of various methods for modeling organometallic chemistry. This differs from most benchmarking studies, as it is common to evaluate theoretical methods by using more computationally expensive calculations to provide the “target” values. With an aim of presenting the ‘best suited functional/functionals’ for calculations involving the metal–ligand bond dissociation enthalpies (BDEs) of organometallic complexes, we utilized a database of 30 experimental metal–ligand bond dissociation enthalpies, and tested for 101 density functionals and 2 ab initio methods, all with a large basis set. We find the most accurate functional is M06 with a mean unsigned error (MUE) of 1.6 kcal mol−1, followed closely by M06L, ωB97XD, PW91PW91 and MPWB95 with MUEs of 1.7, 1.8, 2.0, and 2.1 kcal mol−1 respectively. Other top performers are B3LYP-GD3, BLYP-GD3, PBEPBE, CAM-B3LYP-GD3, CAM-B3LYP-GD3BJ, B3LYP-GD3BJ and MN12L; all predict BDEs with MUEs in the range of 2.2 to 2.5 kcal mol−1. Adding solvent corrections to the gas-phase BDE calculations for these top twelve functionals do not significantly change the MUE value. The well-known and widely used functional B3LYP shows very poor performance for this specific property. However, the empirical dispersion correction to the B3LYP functional has significantly improved its performance in predicting BDEs. It is also worth noting that several modern range-separated functionals predict the bond dissociation enthalpies with an error of 2–3 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...