Copper(i) clusters with bulky dithiocarboxylate, thiolate, and selenolate ligands†
Abstract
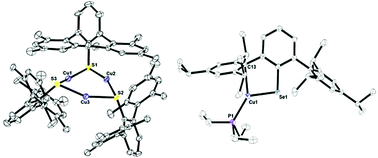
The coordination chemistry of copper has interest due to its use in biological systems as well as for photochemical and medicinal properties. We report the coordination chemistry of copper(I) complexes using terphenyl-based dithiocarboxylate, thiolate, and selenolate ligands. The number of metal ions of the resulting complexes can be tuned by varying the steric properties of the terphenyl ligands, changing the starting material, as well as adding PEt3. In addition, the steric crowding of the terphenyl ligand leads to varying reactivity. For example, while the reaction of carbon disulfide with [Cu(2,6-(Ph)2C6H3)]2 results in an insertion into the copper–carbon bond, no reaction occurs with [Cu(2,4,6-(Mes)2C6H3)], Mes = 2,4,6-Me3C6H2, or [Et3PCu(2,4,6-(Mes)2C6H2)2C6H3]. The synthesis and characterization of new copper(I) complexes using NMR and IR spectroscopy, as well as X-ray crystallography is described.

 Please wait while we load your content...
Please wait while we load your content...