Trinuclear nickel and cobalt complexes containing unsymmetrical tripodal tetradentate ligands: syntheses, structural, magnetic, and catalytic properties†
Abstract
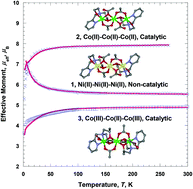
The coordination chemistries of the tetradentate N2O2-type ligands N-(2-pyridylmethyl)iminodiethanol (H2pmide) and N-(2-pyridylmethyl)iminodiisopropanol (H2pmidip) have been investigated with nickel(II) and cobalt(II/III) ions. Three novel complexes prepared and characterized are [(Hpmide)2Ni3(CH3COO)4] (1), [(Hpmide)2Co3(CH3COO)4] (2), and [(pmidip)2Co3(CH3COO)4] (3). In 1 and 2, two terminal nickel(II)/cobalt(II) units are coordinated to one Hpmide− and two CH3CO2−. The terminal units are each connected to a central nickel(II)/cobalt(II) cation through one oxygen atom of Hpmide− and two oxygen atoms of acetate ions, giving rise to nickel(II) and cobalt(II) trinuclear complexes, respectively. Trinuclear complexes 1 and 2 are isomorphous. In 3, two terminal cobalt(III) units are coordinated to pmidip2− and two CH3CO2−. The terminal units are each linked to a central cobalt(II) cation through two oxygen atoms of pmidip2− and one oxygen atom of a bidentate acetate ion, resulting in a linear trinuclear mixed-valence cobalt complex. 1 shows a weak ferromagnetic interaction with the ethoxo and acetato groups between the nickel(II) ions (g = 2.24, J = 2.35 cm−1). However, 2 indicates a weak antiferromagnetic coupling with the ethoxo and acetato groups between the cobalt(II) ions (g = 2.37, J = −0.5 cm−1). Additionally, 3 behaves as a paramagnetic cobalt(II) monomer, due to the diamagnetic cobalt(III) ions in the terminal units (g = 2.53, |D| = 36.0 cm−1). No catalytic activity was observed in 1. However, 2 and 3 showed significant catalytic activities toward various olefins with modest to good yields. 3 was slightly less efficient toward olefin epoxidation reaction than 2. Also 2 was used for terminal olefin oxidation reaction and was oxidised to the corresponding epoxides in moderate yields (34–75%) with conversions ranging from 47–100%. The cobalt complexes 2 and 3 promoted the O–O bond cleavage to ∼75% heterolysis and ∼25% homolysis.

 Please wait while we load your content...
Please wait while we load your content...