Recyclable scavengers for photo-cyclopropanation via an in situ crystallization process†
Abstract
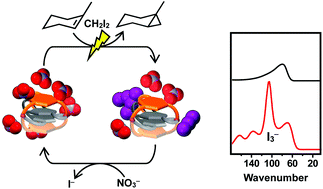
The palladium(II) cyclophane systems, constructed by previously reported proof-of-concept self-assembly, represent a crucial landmark in the field of effective and recyclable scavenging of triiodide (I3−) in the photo-cyclopropanation of alkenes with CH2I2. The scavenger's driving force behind photo-cyclopropanation is the efficient in situ crystallization of triiodide-exchanged species. The exact quantitative photoreaction yields according to the mole ratios of the cyclophane system are impressive. The recycling behavior can be ascribed to the rigidity and stability of the four-layered tripalladium(II)cyclophane.


 Please wait while we load your content...
Please wait while we load your content...