N-Heterocyclic olefins as ancillary ligands in catalysis: a study of their behaviour in transfer hydrogenation reactions†
Abstract
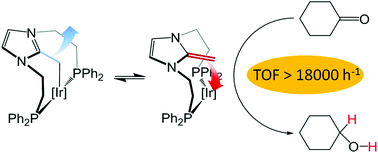
The Ir(I) complexes [Ir(cod)(κP,C,P′-NHOPPh2)]PF6 and [IrCl(cod)(κC-NHOOMe)] (cod = 1,5-cyclooctadiene, NHOPPh2 = 1,3-bis(2-(diphenylphosphanyl)ethyl)-2-methyleneimidazoline) and NHOOMe = 1,3-bis(2-(methoxyethyl)-2-methyleneimidazoline), both featuring an N-heterocyclic olefin ligand (NHO), have been tested in the transfer hydrogenation reaction; this representing the first example of the use of NHOs as ancillary ligands in catalysis. The pre-catalyst [Ir(cod)(κP,C,P′-NHOPPh2)]PF6 has shown excellent activities in the transfer hydrogenation of aldehydes, ketones and imines using iPrOH as a hydrogen source, while [IrCl(cod)(κC-NHOOMe)] decomposes throughout the reaction to give low yields of the hydrogenated product. Addition of one or two equivalents of a phosphine ligand to the latter avoids catalyst decomposition and significantly improves the reaction yields. The reaction mechanism has been investigated by means of stoichiometric studies and theoretical calculations. The formation of the active species ([Ir(κP,C,P′-NHOPPh2)(iPrO)]) has been proposed to occur via isopropoxide coordination and concomitant COD dissociation. Moreover, throughout the catalytic cycle the NHO moiety behaves as a hemilabile ligand, thus allowing the catalyst to adopt stable square planar geometries in the transition states, which reduces the energetic barrier of the process.


 Please wait while we load your content...
Please wait while we load your content...