Mechanisms for dehydrogenation and hydrogenation of N-heterocycles using PNP-pincer-supported iron catalysts: a density functional study†
Abstract
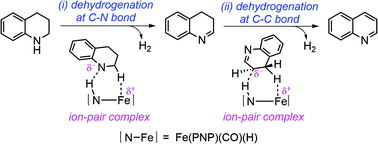
The catalytic dehydrogenation and hydrogenation of N-heterocycles have potential applications in organic hydrogen storage. Recently, Fe(HPNP)(CO)(H)(HBH3) (cp1) and Fe(HPNP)(CO)(H)(Br) (cp2), the iron(II) complexes supported by bis(phosphino)amine pincer (Fe-PNP) (PNP = N(CH2CH2PiPr2)2), have been reported to be the starting complexes which can catalyze the dehydrogenation and hydrogenation of N-heterocycles. The active species were proposed to be the trans-dihydride complexes, Fe(HPNP)(CO)(H)2 (cp4) and Fe(PNP)(CO)(H) (cp3), which can be interconverted. Here, our density functional study revealed that the N-heterocyclic substrate plays a role in the formation of cp4 from cp1, while the tert-butoxide base assists with the formation of cp3 from cp2. The mechanism for cp3 catalyzed dehydrogenation of a 1,2,3,4-tetrahydroquinoline (THQ) substrate to quinoline (Q) involves two main steps: (i) dehydrogenation of THQ to 3,4-dihydroquinoline (34DHQ) and (ii) dehydrogenation of 34DHQ to Q. In each dehydrogenation step, the proton is transferred from the substrate to the N of the PNP ligand of cp3. An ion-pair complex between Fe-PNP and the deprotonated substrate is then formed before the hydride at the adjacent C is transferred to Fe. Notably, the isomerization of 34DHQ to 14DHQ or 12DHQ is not necessary, as the bifunctionality of Fe-PNP in cp3 can stabilize the ion-pair complex and facilitate direct dehydrogenation of the C3–C4 bond in 34DHQ. On the other hand, the mechanism for hydrogenation of Q involves the initial formation of 14DHQ, which can easily isomerize to 34DHQ with the assistance of a tert-butoxide base. Finally, 34DHQ is dehydrogenated to THQ. As the overall energy barriers for cp3 catalyzed dehydrogenation of THQ (+27.6 kcal mol−1) and cp4 catalyzed hydrogenation of Q (+23.8 kcal mol−1) are only slightly different, reaction conditions can be conveniently adjusted to favor either the dehydrogenation or hydrogenation process. Insights into the role of metal–ligand cooperativity in Fe-PNP complexes in promoting the dehydrogenation and the hydrogenation of N-heterocycles should benefit the development of efficient catalysts for organic hydrogen storage.

 Please wait while we load your content...
Please wait while we load your content...