Standard redox potentials, pKas, and hydricities of inorganic complexes under electrochemical conditions and implications for CO2 reduction†
Abstract
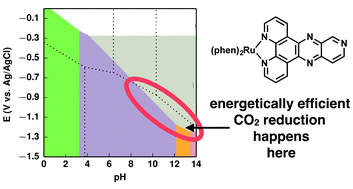
We use computational chemistry to systematically study the thermodynamic stabilities of protonated and reduced intermediate states for Ru(2,2′-bipyridine)3, Ru(1,10-phenanthroline)3, and Ru(phen)2(pyrido[3′,4′:5,6] pyrazino[2,3-f][1,10]phenanthroline) in aqueous solutions. Following our previous studies of aromatic N-heterocycle molecules, we report pKas, standard redox potentials, and hydricities as well as computationally derived Pourbaix diagrams that show which states would be thermodynamically stable at different conditions of pH and applied potential. Locations of added electrons within ligands and complexes after reductions are also shown with electron density difference plots. As with other aromatic N-heterocycle molecules implicated in CO2 reduction, we find that several of the boundary lines from the Pourbaix diagrams are in close proximity to the thermodynamic redox potentials for CO2 electroreductions, making them thermodynamically appropriate for energetically efficient hydrogen shuttling.

 Please wait while we load your content...
Please wait while we load your content...