A novel fluorescence “on–off–on” chemosensor for Hg2+via a water-assistant blocking heavy atom effect†
Abstract
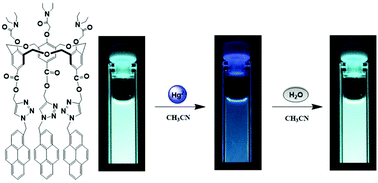
Upper rim pyrene-functionalized hexahomotrioxacalix[3]arene L was synthesized via Click chemistry, and its fluorescence behaviors toward several common metal cations were investigated. L exhibited a significant fluorescence quenching response to Hg2+ in CH3CN solution, which was unaffected by the coexistence of other competitive metal cations. Thus, L can be utilized as a highly selective and sensitive fluorescent chemosensor for Hg2+ with a detection limit in the nM level. Interestingly, the quenched fluorescence emission can be successfully revived upon the addition of water. In this process, the heavy atom effect of Hg2+ can be blocked by further coordination of a water molecule and resulted in the revival of the fluorescence emission of L/Hg2+ complex. Particularly, other polar solvents such as CH3OH and CH3CH2OH also have the ability to revive the fluorescence emission of the L/Hg2+ complex, but on a much smaller scale than observed for H2O. The heavy atom effect and blocking thereof were demonstrated within the same system by the use of a C3-symmetric homooxacalix[3]arene scaffold. The present studies provided further evidence for the blocking heavy atom effect.

 Please wait while we load your content...
Please wait while we load your content...